Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Time-resolved laser-induced fluorescence spectroscopy (TRLFS) is a very sensitive, versatile spectroscopic method used to follow the complex formation of Eu(III) and Cm(III) in solution at sub micromolar concentrations by recording fluorescence emission spectra1. In order to gain further structural information the fluorescence lifetime is determined. For our research the dependency of the fluorescence lifetime on the nature of the coordinating ligand is used. It is found that the fluorescence lifetime increases with decreasing number of quenching molecules in the first coordination sphere. Hereby both coordinated water2,3 and alcohol4 molecules cause fluorescence quenching, mainly by energy transfer via excitation of vibrational modes of OH functions. In this report we present fluorescence lifetimes of solvated Eu(III) and Cm(III) ions.
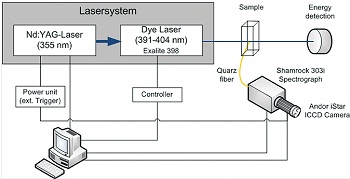
Figure 1. Schematic view of the TRLFS setup.
Eu(ClO4)3 is purchased from Alfa-Aesar and used as received. A 2.1·10-5 molL-1 Cm(ClO4)3 solution in 0.1 molL-1 HClO4 which was obtained from a 252Cf source and diluted to 6.7·10-6 molL-1. The isotopic mass distribution of the Cm(ClO4)3 solution is 89.7 % 248Cm, 0.1% 247Cm, 9.4% 246Cm, 0.1% 245Cm, 0.3% 244Cm, and 0.4% 243Cm according to alpha spectroscopy and ICP-MS. The Eu(ClO4)3 solution is prepared by adding 20 µL of a 9.0·10-4 molL-1 aqueous stock solution of Eu(ClO4)3 in 0.01 molL-1 HClO4 into 980 µl 0.01 molL-1 HClO4 resulting in a 1.8·10-5 molL-1 solution of Eu(III). The Cm(ClO4)3 solution is prepared by adding 15 µL of a 6.7·10-6 molL-1 aqueous stock solution of Cm(ClO4)3 in 0.01 molL-1 HClO4 into 985 µL 0.01 molL-1 HClO4 resulting in 1.0·10-7 molL-1 solution of Cm(III). TRLFS measurements are performed using a Nd:YAG-pumped dye laser system [Surelite II laser (Continuum), NARROWscan D-R dye laser (Radiant Dyes Laser Accessories)].
For Eu(III) excitation a wavelength of 394.0 nm and for Cm(III) a wavelength of 396.6 nm was used. The emission spectra are recorded at 90° to the exciting laser beam. A Shamrock SR-303i spectrograph (Andor), equipped with a 300, 900 and 1200 lines/mm grating turret is used for spectral decomposition. The fluorescence emission is detected by an ICCD camera (Andor iStar Gen III, A-DH720-18F-O).
Figure 2. Eu(III Ffluorescence
Rayleigh scattering and shortlived fluorescence of organic ligands is discriminated by a delay time of 1.0 µs before fluorescence light is recorded. The gate width of the intensifier is set to 1 ms, thus integrating the interval of fluorescence decay after the delay. The quartz cuvette is temperature controlled at T = 25 °C. A schematic view of the experimental setup is shown in fig. 1.
The emission spectrum of the solvated Eu(III) species ([Eu(H2O)9]3+) in 0.01 mol L-1 HClO4 shows one broad band with an emission maximum at 593.4 nm for the 5D0?7F1 and a emission maximum at 617.4 nm for the broad 5D0?7F2 transition.5 An example of the orange coloured Eu(III) fluorescence is shown in fig.2.
![Figure 3. Development of the solvated Eu(III) emission band as function of the delay time, [Eu(III)] = 1.8·10-5 mol L-1 in 0.01 mol L-1 HClO4.](https://www.oxinst.com/assets/uploads/learning/fluorescence-lifetimes/sp_3_AP_3.jpg)
Figure 3. Development of the solvated Eu(III) emission band as function of the delay time, [Eu(III)] = 1.8·10-5 mol L-1 in 0.01 mol L-1 HClO4.
The fluorescence lifetime of the solvated Eu(III) is obtained by recording emission spectra at different delay times between the laser pulse and the beginning of the detection.
The development of the solvated Eu(III) emission band as function of the delay time is shown in fig. 3.
![Figure 4. Development of the solvated Cm(III) emission band as function of the delay time, [Cm(III)] = 1.0·10-7 mol L-1 in 0.01 mol L-1 HClO4.](https://www.oxinst.com/assets/uploads/learning/fluorescence-lifetimes/sp_3_AP_4.jpg)
Figure 4. Development of the solvated Cm(III) emission band as function of the delay time, [Cm(III)] = 1.0·10-7 mol L-1 in 0.01 mol L-1 HClO4.
The evolution of the Cm(III) fluorescence spectrum in 0.01 mol L-1 HClO4 resulting from the 6D’7/2?8S’7/2 transition is shown in Figure 4. The solvated metal ion species, [Cm(H2O)9]3+, displays a broad emission band at 593.8 nm.1,3 Fluorescence lifetime data of the solvated Cm(III) is obtained in an analogue procedure as for Eu(III).
For both metal ions the fluorescence lifetime t, is obtained by fitting the integrated intensity I(?) corresponding to a delay time t after the laser pulse according to I(?) = I0(?) exp(-t/t).
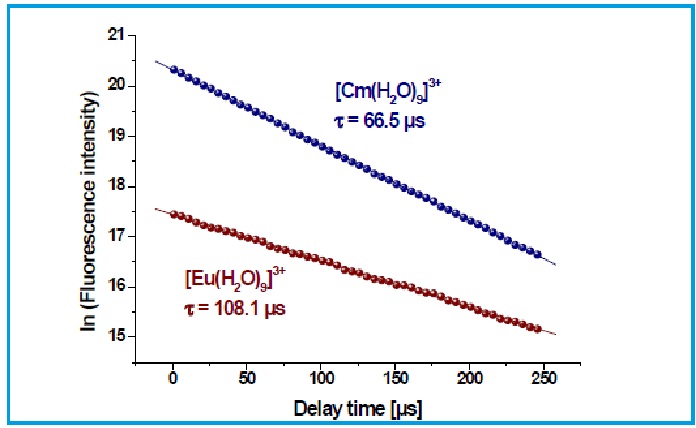
Figure 5. Decay of the fluorescence intensity of solvated Eu(III) and Cm(III) ions in 0.01 mol L-1 HClO4.
For Eu(III) a fluorescence lifetime of t = 108.1 µs and for Cm(III) a fluorescence lifetime of t = 66.5 µs is determined. Figure 5 shows the monoexponential decay of the fluorescence intensity for [Eu(H2O)9]3+ and [Cm(H2O)9]3+.
The new ICCD camera time-resolved spectroscopy setup allows very easy change between different gratings and a much lower detection limit, allowing a significant saving of the applied Eu(III) and Cm(III) material. The standard concentration of Eu(III) solutions used for our research is decreased from 2.0·10-5 mol L-1 to 1.0·10-6 mol L-1 and the concentration of applied Cm(III) solutions from 2.0·10-7 mol L-1 to 1.0·10-8 mol L-1 respectively. Furthermore the program interface is significantly more user friendly. These improvements result in an entirely happy user (see fig. 6).

Figure 6. A happy user.
Acknowledgements
We thank Nicole Bauer, Christian Adam and Janina Kasperidus for artwork used in this report.
Contact
Dr. Björn B. Beele, Dipl.-Chem. Christian M. Ruff,
Karlsruhe Institute of Technology,
Institute for Nuclear Waste Disposal (INE),
P. O. Box 3640, 76021 Karlsruhe, Germany
Mail: : Bjoern.Beele@partner.kit.edu
Christian.Ruff@kit.edu
Web: www.ine.kit.edu
Literature
1. R. Klenze, J. I. Kim, H. Wimmer,
Radiochim Acta 1991, 52-3, 97-103.
2. S. Trumm, A. Geist, P. J. Panak, T. Fanghänel,
Solvent Extr. Ion Exch. 2011, 29, 213-229.
3. T. Kimura, G. R. Choppin, Y. Kato and Z. Yoshida,
Radiochim. Acta 1996, 72, 61-64.
4. W. D. Horrocks, D. R. Sudnick,
J. Am. Chem. Soc. 1979, 101, 334-340.
5. J. C. G. Bünzli, J. R. Yersin,
Inorg. Chem.1979, 18 (3), 605-607.
