Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
FRET (sometimes called Förster Resonance Energy Transfer) enables the proximity of two fluorophores to be determined. FRET is one of a number of single molecule techniques such as TIRF, SIM and super-resolution localisation that have gained popularity in recent years. Resonance energy transfer occurs only over very short distances, typically within 10 nm, and involves the direct transfer of excited state energy from the donor fluorophore to an acceptor fluorophore as an alternative to fluorescence emissive decay from the donor. Upon transfer of energy, the acceptor molecule enters an excited state from which it decays emissively (always of a longer wavelength than that of the acceptor emission). By exciting the donor and then monitoring the relative donor and acceptor emissions, either sequentially, or simultaneously, one can determine when FRET has occurred and at what efficiency.
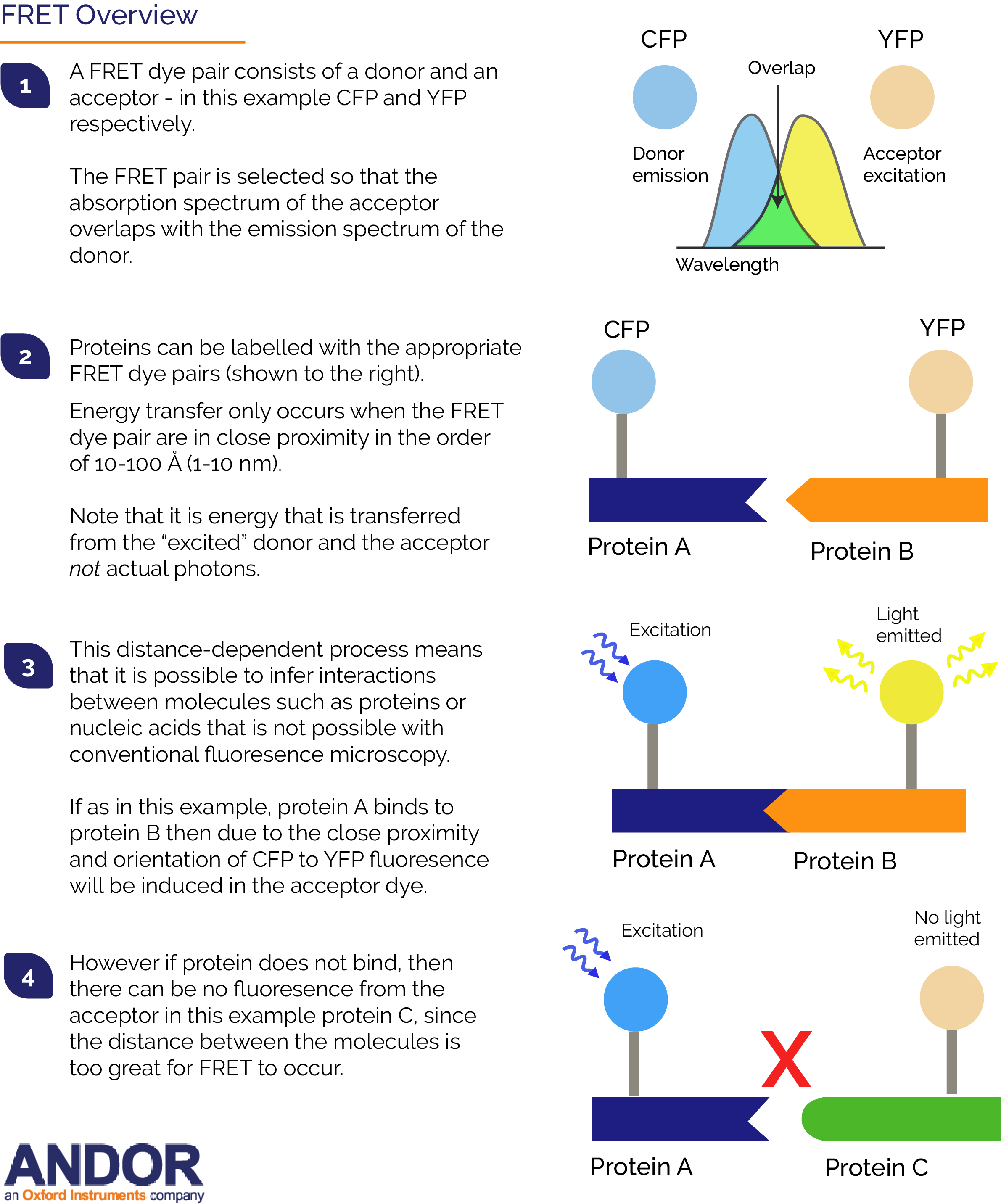
Fluorophores can be employed to specifically label biomolecules of interest and the distance condition for FRET is of the order of the diameter of most biomolecules (1-10 nm). This means that FRET can therefore be exploited to determine when, and where, two or more of these labelled biomolecules (typically proteins) interact within their physiological surroundings. The FRET signal corresponding to a particular location within a microscope image provides an additional distance accuracy surpassing the optical resolution (~0.25 mm) of the light microscope. Aside from spatial proximity, for efficient FRET to take place the FRET dye pair must also exhibit significant overlap of the donor's excitation spectrum with the acceptor's absorption spectrum. It is this characteristic that constitutes one of the experimental paradoxes of FRET:
In practice, this can achieved using short bandpass filters that collect light from only the shorter wavelength side of the donor emission and the longer wavelength side of the acceptor emission. This can limit somewhat the photon flux from both donor and acceptor during a typical exposure, especially when we bear in mind that these measurements are best performed under conditions of reduced excitation power, such that we do not accelerate the rates of bleaching. This means that ultra-sensitive detectors are required for FRET experiments.
Example FRET Dye pairs include:
The profile for the CFP-YFP FRET dye pair is shown below:
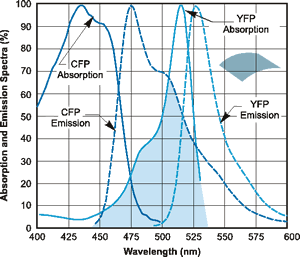
Absorption and emission spectral profiles of the CFP-YFP FRET pair.
Andor's iXon EMCCD cameras, whether as a key component of the Dragonfly live cell confocal imaging platform, or within another confocal system are well-established detector solutions for FRET imaging. EMCCD enables high resolution, high signal-to-noise (S/N) determination of FRET interactions throughout the imaged area or volume of the cell and helps to address the low photon levels present when narrow-band filters are used. When combined with a careful choice of filter sets it ensures a high integrity of FRET data. Since EMCCDs overcome the noise floor detection limit at any readout speed, molecular interactions can be followed dynamically with high accuracy. Furthermore, excitation power can often be reduced meaning that phototoxic and photo-bleach effects are minimized so that molecular interactions can be followed for much longer periods. For further information on detector selection for single molecule studies please view the article What is the best Detector for Single-Molecule Studies?
1. Oh H-K et al. (2019) Rapid and Simple Detection of Ochratoxin A using Fluorescence Resonance Energy Transfer on Lateral Flow Immunoassay (FRET-LFI)Toxins 11(5), 292; https://doi.org/10.3390/toxins11050292
2. Hu J. et al (2018) Combining gold nanoparticle antennas with single-molecule fluorescence resonance energy transfer (smFRET) to study DNA hairpin dynamics Nanoscale,10, 6611-6619 10.1039/C7NR08397A
3. Chaurasiya K.R., Dame R.T. (2018) Single Molecule FRET Analysis of DNA Binding Proteins. In: Peterman E. (eds) Single Molecule Analysis. Methods in Molecular Biology, vol 1665. Humana Press, New York, NY
4. Jung, S. et al (2018), Real‐Time Monitoring of the Binding/Dissociation and Redox States of a Single Transition Metal Ions. Bull. Korean Chem. Soc., 39: 638-642. doi:10.1002/bkcs.11443
Date: N/A
Author: Alan Mulllan
Category: Application Note
