Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Intracellular pH is important in many functions that take place in a cell. For example, pH affects protein structure and the function of lysosomes, mitochondria, and other organelles. Changes in energy metabolism also often correlate with pH changes, and so scientists would like to monitor ATP and pH in a cell simultaneously.
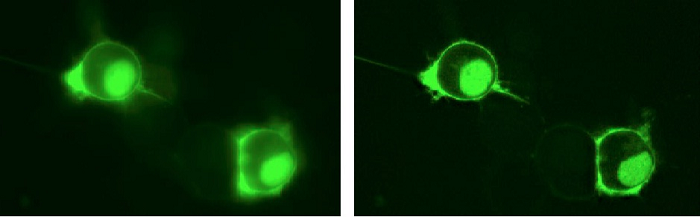
Figure 1a. The researchers transfected Neuro-2A cells with a single fusion construct of pHRed-Perceval targeted to the plasma membrane and the nucleus. The Andor Revolution DSD was used to collect both widefield and confocal signals at a single z-plane through the center of the cells using a 40X 0.95NA air objective. The widefield image is on the left, and the confocal image is seen on the right. Only the green Perceval emission is represented in the image.
The Andor Revolution DSD confocal microscope is helping Dr. Gary Yellen’s lab at Harvard Medical School develop genetically encoded fluorescent sensors for monitoring metabolism of single cells. “We are interested in sub-cellular compartmentalization of metabolites, and the optical sectioning afforded by the DSD allows us to assess the distribution of our sensors when they are genetically targeted to different cellular locations (e.g., plasma membrane, mitochondria, etc; Figures 1a and 1b),” he says.
Dr. Yellen’s research team recently developed a genetically encoded pH sensor that could be used with GFP-based ATP sensors (Figure 1.) This type of probe was needed to monitor ATP and pH changes in a cell and to correct for the pH sensitivity of ATP sensors. They used the red fluorescent protein mKeima as a basis to engineer a genetically encoded ratiometric pH sensor they named pHRed. The sensor has a fluorescence emission peak at 610 nm and dual excitation peaks at 440 and 585 nm that allow ratiometric imaging.
Postdoctoral research fellow Mathew Tantama imaged energydependent changes in cytosolic and mitochondrial pH with the Revolution DSD, demonstrating pHRed’s capability to monitor intracellular pH in living cells. “The Andor Revolution DSD offered us a practical, cost-effective solution for optical sectioning with the flexibility of a white light source,” Dr. Yellen says. The microscope’s broadband prefiltered light source and user-selectable filters gave the researchers access to a wide variety of excitation wavelengths. “During the process of sensor development, color requirements often change, making the fixed wavelengths of laser-based systems constraining or cost-prohibitive for an individual lab,” he adds. The unique optical architecture of the Revolution DSD enables capture of both conventional and confocal fluorescence images simultaneously. This feature provides further flexibility for the researchers who can choose between the sensitivity of widefield and the resolution of confocal imaging according to conditions and experimental requirements.
 <
<
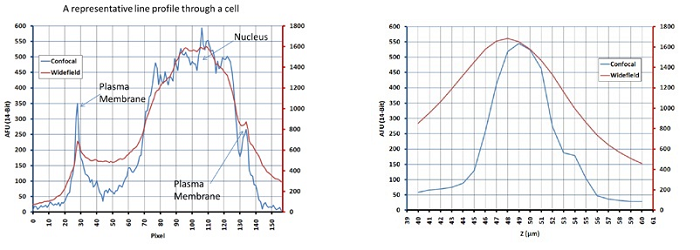
Figure 1b. The widefield and confocal signals were simultaneously acquired with the Andor DSD system, and data are shown for a representative line profile through a cell (left) and a representative axial intensity profiled through the nucleus (right). Subcellular compartments such as the plasma membrane and nucleus are better defined in the confocal image compared to widefield, allowing more spatially accurate measurements. The wide field mode of the DSD is useful when spatial variation is minimal but more sensitivity is required.
The researchers transfected the pHRed sensor and the mVenusbased Perceval ATP/ADP sensor into live cells. For intensity ratio imaging they imaged the cells on an inverted microscope equipped with a Revolution DSD system controlled by Andor iQ software. Ratio imaging of the two sensors used excitation filters 445/20 nm, 482/18 nm, or 578/16 nm filters. The DSD unit contained a 59022bs dichroic, 492 nm short pass, 490 nm short pass, and 590 nm short pass filters. Emission light was passed through 525/39 nm or 629/56 nm filters. They typically acquired images every 10 seconds with 2x2 binning and 50 to 400 ms exposure times using a 20X/0.75NA objective. The speed performance of the Revolution DSD allowed imaging of several wavelengths at multiple XY positions while maintaining an adequate temporal resolution.
After capturing the images, they subtracted background and bleedthrough of Perceval fluorescence into the pHRed channel before calculating ratios. At the shared wavelength of 445 nm there was little bleedthrough during excitation. The researchers calculated pixel-by-pixel ratios, identified regions of interest around cells, and calculated averaged measurements using thresholding with ImageJ software. They found that pHRed’s intensity ratio responds with an apparent pKa of 6.6 and a greater than 10-fold dynamic range.
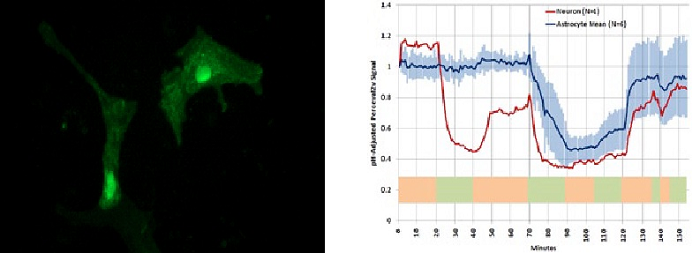
Figure 2. Mouse primary astrocytes (pictured above) as well as neurons were transfected with a single fusion construct of pHRed-Perceval targeted to the plasma membrane and nucleus. Data acquired with the DSD confocal microscope (graph above) demonstrates that the sensors report neuronal response to glutamate challenge (t = 20 to 40 min) with a decrease in ATP/ADP but astrocytes did not. All cells responded to lowering of extracellular glucose (t = 70 to 120 min). In this experiment, images in 4 separate color channels at 4 stage positions were taken at each time point with only the green Perceval emission being represented in the image.
“This is the first ratiometric sensor based on a single red fluorescent protein that can be used in multiple imaging modalities,” Dr. Yellen says. “There are several other genetically-encoded pH sensors that utilize the blue to yellow color range or that provide intensity-based readouts only. Our red-colored pH sensor can be used in conjunction with other genetically-encoded sensors with the possibility of minimal color channel crosstalk.”
