Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Techniques for the study of intracellular ions are used widely in biology, for example, to identify spatial variations in calcium levels within living cells, to measure the concentrations of intracellular ions including cell pH, and to monitor how these concentrations change with time. Monitoring intracellular ion changes is vital for our understanding of signaling and functional pathways in cellular systems, central to many fundamental processes such as muscle contraction as well as synaptic nerve signal transmission. Such measurements have been central to a range of research programs, including drug discovery research. Calcium channels are representative of an important class of cell membrane molecules known as ion channels. As these open or close, e.g. in response to extracellular messenger molecules, intracellular ion concentrations can also change, altering how the cell behaves. Therapeutic agents that act directly on these ion channels may provide effective treatments for many diseases. For example, drugs that target calcium channels, are useful in treating a variety of cardiovascular disorders.Fluorescent dyes are designed to have affinity to specific ions; for example, FURA-2 is specific to calcium ions. When they bind to an ion, their absorption or fluorescent properties are altered. The principle of ion concentration determination is such that, when the free ion concentration changes, the equilibrium between free indicator dye and ion-bound dye also changes, resulting in a change in the photophysics of the indicator dye.Ratiometric microscopy is one approach for such measurements - by quantitatively interpreting the changes in fluorescent properties by looking at both bound and unbound dye ratio, the concentration of the ion being investigated can be measured. However, there are some common non-ratiometric dyes also, such as Fluo-4.
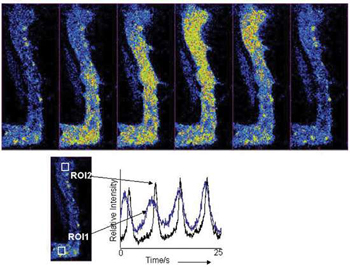
Selected fluorescence images, taken with the Revolution 488, from a 1500 frame kinetic series at 30 frames/sec, showing the progression of a Ca2+wave as it progresses though a rabbit urethral muscle cell.Kinetic plots derived from offset ROIs within the cell show temporal progression of the Ca2+flux. Using the same field of view and no binning, frame rates were then pushed to 120fps for better temporal resolution of events. Courtesy of Dr Mark Hollywood, Smooth Muscle Research Centre, Dundalk Institute of Technology.
In many tissue types, ion flux changes occur rapidly, very often in the low millisecond time domain. As such, ion-binding fluorescence dyes tend to have rapid responses, enabling changes in concentrations of free ions to be imaged with high temporal resolution, requiring a means of fast quantitative imaging of these fluorescence intensity changes. Ultimately however, we are practically limited by the binding constants of these dyes – the rate of imaging does not need to not exceed the time taken for dyes to diffuse, bind and respond to free ions, irrespective of how rapidly the ion concentration changes. One is better determining this binding constant of the dye, and using an exposure time suited to this. Using too short an exposure time adversely affects signal to shot noise ratio, the ultimate limiting detection phenomenon (which can make your image look grainy and unresolved), as would be the case even in the presence of a "perfect" photon detector (100% Quantum Efficiency (QE) + zero noise floor).Another factor that must be taken into consideration is the concentration of ion dye used. Ideally, lowest possible concentrations of dye are used as indicators of free ion concentrations, such that the dye binds to negligible amounts of ions as compared to the total quantity of the ion species in question, and so it does not significantly alter the statistical equilibrium of bound and free ions. The remedy is to lower the concentration as far as possible whilst still maintaining an adequate signal to noise ratio for the exposure time (and therefore frame rate) required.
Andor solutions for Ion Signalling Microscopy
Andor's EMCCD technology, whether as a key component in our Revolution confocal live cell imaging system, or as a "stand-alone" EMCCD + iQ imaging software solution, is the proven solution for ultra-sensitive rapid imaging of ion concentrations. The Signal to Noise (S/N) achievable at rapid frame rates is ideally suited to intracellular visualization and measurement of common ion-binding dyes such as the calcium-specific Fluo-4. EMCCD technology enables readout noise to be completely negated, even at very fast readout rates, ideally suited to temporal requirements of calcium transient processes, such as calcium sparks or waves. The iXon Ultra 897 with a pixel format of 512 x 512 is capable of 595 frames/ sec using an ROI of 128 x 128 with the 'Cropped Mode' feature. In addition, the iXon3 860 back-illuminated is capable of > 500 frames/sec @ 128 x 128 pixel format. Through use of EMCCDs, calcium dye concentrations have also been reduced, enabling propagation of calcium waves along the full length of elongated smooth muscle cells for example. High sensitivity also enables excitation power to be attenuated. Through minimization of excitation powers, whether laser or lamp based, photobleaching rates of the dyes can be dramatically reduced. In living cell environments, these "milder" excitation conditions also reduce the phototoxic effects that would otherwise kill the living cells, enabling them to be studied over longer periods of time.
Recently, with the introduction of sCMOS technology, researchers are finding the large field of view, fast speeds and low noise a benefit for their ion imaging experiments. Andor have a choice of sCMOS detectors available to suit a wide range of ion signalling applications. Andor's Neo and Zyla 5.5 have a 5.5 megapixel sensor with a 'True Global Shutter' mode which is required if one wants to image multiple signalling events simultaneously in one image. Alternatively, Zyla 4.2 has a 4.2 megapixel sensor with a higher QE offering more sensitivity for measuring ion concentrations. All sCMOS cameras from Andor simultaneously offer a large field of view (5.5 MP or 4.2 MP), extremely low noise (< 1 e-), wide dynamic range (33,000:1) and very fast frame rates (100 fps sustained).
