Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Since the dawn of modern medicine, human biological material has been collected for a variety of purposes. Should the material be collected for diagnostic purposes, it is often possible to be satisfied with a relatively short shelf-life. However, many unique samples are collected and stored for later use and we have witnessed rapid growth in the demand for biobanking in reproductive and regenerative medicine, tissue engineering, stem-cell and cell biology, and pharmaceutical research. In almost all cases, blood and plasma being the most common exceptions, biological integrity throughout the long-term storage is ensured by cryopreservation at temperatures below -137°C.
Introduction
These samples may be unique and virtually irreplaceable, but many of these specimens have been lost, and continue to be lost, through thermal damage. Any testing for physiological and genetic soundness before their use is likely to render them unfit for subsequent use in medical procedures. This in turn raises the issue of an increasing number of samples entering long-term storage without the possibility to check that thermal damage has not occurred during storage and before eventual use. A recently published paper by Professor Heiko Zimmermann and his colleagues from Saarland University’s Biophysics and Cryotechnology department lays out a possible framework to address this issue [1]. It describes a powerful new technique that offers the ability to not only verify the absence of physical and chemical change due to devitrification inside the sealed sample, but also automate periodic, non-invasive monitoring of entire biobank samples.
Biobanks and Cryopreservation
Biobanks rely on the fact that aqueous samples are considered to be stably cryopreserved upon cooling to below -137°C, as this is the glass transition temperature (Tg) of amorphous water. Chemical and biological activity, and diffusion also virtually stop below this temperature.
Ice crystal formation does not occur below Tg, which is particularly significant with respect to vitrified samples that have been solidified in a metastable, amorphous (glassy) state by sudden cooling. If ice forms in vitrified samples due to transient infringing of the Tg (devitrification), ramification of the crystals will lead almost inevitably to the loss of the complete sample. Transient warming incidents sufficient to cause ice crystal formation are possible in biobanking practice, for example when moving single specimens or entire sample collections. But, the potentially ruinous effects on the sample of an interruption in the cold chain are not currently detectable before rewarming, i.e. ahead of specimen use. Until now, technical solutions for monitoring the integrity of the sample in situ have not been available and there has been no established technique for retrospective proof of physical and chemical changes inside the sealed sample.
Optical monitoring
The team from Saarland University has demonstrated a powerful, Raman-scattering based technique for noninvasively monitoring and investigating cryopreserved samples. In particular, they have confirmed unambiguously the detection of ice crystals in vitreous samples in situ at temperatures below -120°C.
They focused on the OH-stretching band to investigate whether the sample water is amorphous or crystalline. Conditions in cryogenic storage and typical slow freezing conditions were mimicked using a cooling stage capable of a rate of -1K/min. At the heart of the Raman detection system is a fibre-borne Shamrock 303i imaging spectrograph from Andor coupled to an iDus back-illuminated deep-depletion (‘BRDD’) CCD camera in order to access the longer illumination wavelength if cellulat autofluorescence became an issue. According to Professor Zimmermann, a bespoke Labview-based software was developed to allow data capture, export and processing.

Figure 1: A confocal Raman micrograph (65*65 μm2, colour-coded for chemical compounds) of the phase texture of frozen biobanking media at -50°C. Ice crystals are visible as dark areas separated by fluid channel enriched in DMSO (a well-known cryoprotective agent) highlighted in green. The red structures were identified as hydrohalite – a crystalline rock salt hydrate only stable at sub-zero temperatures.
Zimmerman’s team created chemical maps of the samples using confocal Raman microscopy. Each pixel of the image contains an entire Raman spectrum, and images of arbitrary spectral channels can be generated to display isolated, useful molecular vibrations. In this way, Raman microscopy can measure and map the concentration as well as the chemical state of the main constituents of a sample. Figure 1 is a confocal Raman micrograph of the phase texture of frozen biobanking media at -50°C. Ice crystals are visible as dark areas separated by fluid channels enriched in DMSO (green) with hydrohalite, a crystalline rock salt hydrate only stable at sub-zero temperatures, visible as red.
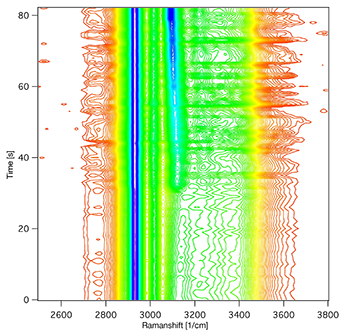
Figure 2: A contour diagram showing the emergence of the ice band due to devitrification upon rewarming a sample beyond the glass transition temperature at about -125°C.
To resemble typical slow freezing conditions, the team cooled a single suspended human mesenchymal stem cell (hMSC) down to -120°C at a rate of -1K/min in a PBS solution containing 10% DMSO and collected the Raman images. Then, in order to prove the concept of monitoring recrystallization of vitrified samples during cold storage and the possibility of thermal damage during routine biobanking operations, the team mimicked a measured typical temperature profile of a sample vial while a neighbouring vial was withdrawn from the cryocontainer. They started the monitoring process at -141°C and heated the sample at a rate of 2 K/min. Until a temperature of -127°C, the spectra show no significant changes but crystallization onset was detected at approx. -127°C and completed at approx. -122°C. Figure 2 is a contour diagram showing the emergence of a characteristic ‘ice band’ due to devitrification upon rewarming a sample beyond the glass transition temperature at approx. -125°C. Furthermore, the ‘ice band’ was reproducibly detected on crystallization of samples of various compositions, including cell suspensions. In this way, Zimmermann and his co-workers have demonstrated how the fatal effect of devitrification – ice formation in vitreous samples – can be unambiguously detected by Raman spectroscopy below -120°C.
The potential in Biobanking
Raman probe screening of cryopreserved samples could be the first stage in establishing a recognised technique for retrospective proof of the absence of physical and chemical changes inside the sealed sample due to devitrification. Furthermore, this technique allows a differentiation between cryoprotective agents (CPAs) as well as freezing protocols, such as vitrification or slow freezing. Moreover, in principle the technique is capable of detecting any other chemical change in samples and has the potential to be a powerful method for contactless monitoring of the status of cryopreserved samples during storage.
Implementation of the concept could lead the way to automated monitoring of samples under cryogenic storage in the future, although storage technology would need to evolve to allow transport of samples to an optical interface inside the cryo-tank. It is possible that this degree of complexity and cost will only be considered for very valuable samples.
In the short term, however, the authors have shown that it would be possible to take a fingerprint spectrum of every freshly preserved sample and use it as a reference to periodically confirm sample integrity during storage and upon eventual release. The research has also shown that their confocal setup and the present fibre probe already allow measurements through turbid container walls, such as Teflon straws, and the team is working on the development of a multispot confocal probe for workbench applications.
They suggest that biobanking practice incorporates the pre- and post-storage sample characterisation in cryogenic temperature workbenches to check for chemical changes during the conservation and for ice formation in vitrified samples. A quality assessment for sample transportation can also be performed this way. They also suggest that Raman spectroscopy could be used to develop catalogue references for the quality management of cryopreserved samples and that comparison of existing samples with catalogued spectra may enable retrospective sample analysis.
References
