Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Cell migration is essential during development, tissue regeneration, and metastasis. As cells move, they attach to the underlying extracellular matrix using focal adhesions. Assembly and disassembly of these adhesions must be tightly controlled so that cells can move and migrate in a coordinated manner. Dr. Torsten Wittmann’s lab at the University of California-San Francisco uses advanced microscopy techniques, together with biochemistry and molecular biology approaches, to study the complex protein dynamics involved in cell migration.
In a recent study, the researchers used confocal image analyses to gain insight into how focal adhesion assembly and disassembly is locally controlled in migrating epithelial cells. Based on their findings, which are reported in a 2014 Nature Cell Biology paper, the researchers propose a new mechanism by which microtubules participate in focal adhesion dynamics.
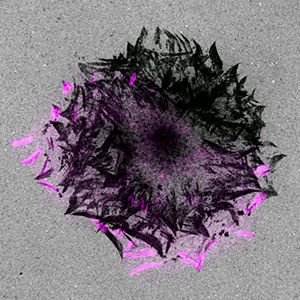
Figure 1. Focal adhesion turnover (paxillin-mCherry: purple) in spreading keratinocytes leaves behind footprints of proteolytic degradation of the extracellular matrix. The fluorescently labeled gelatin is grey. Images courtesy of Torsten Wittmann, UCSF.
For this research, Dr. Wittmann’s team used a Borealis-modified Yokogawa CSU-X1 spinning disk confocal head on a Nikon T1 inverted microscope. Their optical set up also included an Andor Clara cooled interline CCD camera and four 100 mW diode-pumped solid-state lasers at 442 nm, 488 nm, 515 nm, and 561 nm. They imaged intracellular proteins, tagged with fluorescent proteins, in migrating epithelial cells with focal adhesions showing highly coordinated turnover. The enhanced field flatness achieved with the Borealis introduces less variability into fluorescence intensity measurements across the field of view, which was important during their analysis of focal adhesion dynamics as cells move across the field.
Studies have shown that microtubules regulate the focal adhesion disassembly and turnover required for migration, but the specifics of how this turnover is locally controlled in migrating cells isn’t understood. The researchers took a closer look at how CLASP proteins are involved in this process since these plus-end tracking proteins (+TIPS) have been shown to promote the stability of peripheral microtubules.
CLASP helps link microtubules to focal adhesions
Using the Borealis-modified spinning-disk confocal microscope, the researchers conducted a series of image analysis experiments that revealed that CLASP-decorated microtubule clusters assemble around mature focal adhesions, and that CLASP proteins help tether microtubules to the focal adhesions. They acquired time-lapse images of paxillin-mCherry and eGFP-CLASP2 turnover for 3 hours at 3-minute intervals. Paxillin is known to be targeted to focal adhesions. The researchers then examined focal-adhesion-associated extra-cellular matrix degradation in non-transformed migrating epithelial cells plated on fluorescently labeled gelatin to see if this process might control focal adhesion turnover. They acquired spinning disk confocal microscopy images of these cells and selected regions of interest within microns of the cell edge. They thresholded the regions of interest to include areas of Alexa 488 gelatin degradation and normalized the degradation area to the total area of the region of interest to calculate the percentage of degradation.
After 23 hours, the researchers saw punctate extracellular matrix degradation under the cell center as well as peripheral areas of degradation that sharply delineated focal adhesions. They observed a pattern of footprints that showed many generations of focal adhesions as the cell spread (Figure 1). Time-lapse microscopy and analysis of fluorescence intensities revealed strong spatial and temporal correlation between turnover of focal adhesions (paxillin labeled with mCherry) and Alexa 488 gelatin degradation. In CLASP-depleted cells, gelatin degradation was seen in the cell center but not in areas correlating with focal adhesions, showing that CLASPs are required for local focal adhesion-associated extracellular matrix degradation.
Is CLASP involved in focal adhesion exocytosis?
Further imaging experiments were conducted to better understand how CLASPs could guide local activity of the membrane-associated matrix metalloprotease MT1-MMP. The researchers hypothesized that intracellular vesicle transport to and/or from focal adhesions along microtubule tracks may play a role in CLASP-mediated focal adhesion disassembly. However, confocal imaging revealed that CLASP clusters were probably not involved in endocytosis-mediated focal adhesion turnover.
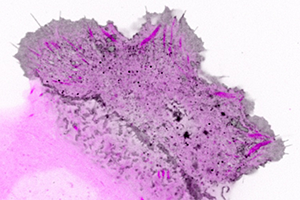
Figure 2. Confocal imaging of the intracellular dynamics of membrane-bound EGFP-MT1-MMP matrix metalloprotease (grey) and focal adhesion dynamics (purple) in a migrating human keratinocyte. This analysis revealed enriched membrane-bound EGFP-MT1-MMP along mature focal adhesions. For better visualization, contrast is inverted in this image.
Dr. Wittmann’s research team then decided to test whether the CLASP-stabilized microtubule tracks that connect to focal adhesions could instead serve as anterograde transport pathways that aid the degradation of the extracellular matrix through focal adhesion exocytosis involving MT1-MMP. For this analysis they used confocal microscopy to examine eGFP-labeled MT1-MMP in relation to the turnover of focal adhesions in which paxillin was labeled with mCherry.
Thanks to the extra excitation light provided using the Borealis module, the researchers could use shorter exposure time and thus significantly reduce motion blur artifacts when imaging fast-moving intracellular structures such as eGFP-MT1-MMP vesicles (Figure 2, Video). However, they point out the importance of using direct hardware triggering of excitation light shutters by the camera to limit excessive phototoxicity at high light intensities. This image analysis showed that along mature focal adhesions, eGFP-MT1-MMP was bound to the membrane and found in smaller intracellular vesicles. Subsequent TIRF microscopy experiments revealed transport, docking, and sudden disappearance of eGFP-MT1-MMP vesicles near focal adhesions, which suggested exocytosis was taking place.
To test whether CLASPs mediate microtubule interactions with focal adhesions, the researchers compared focal adhesion dynamics in control cells with that of cells they generated to have depleted CLASP function. In the CLASP-depleted cells, the microtubules did not move toward the focal adhesions, indicating that CLASPS help to tether microtubules to the focal adhesions.
Overall, the research showed that microtubule-associated CLASP tethers microtubules to focal adhesions, and the work provides the first direct evidence of targeted exocytosis in migrating cells. Based on their findings, the researchers propose that these microtubules establish a targeted secretory pathway directed to focal adhesions. They think that this pathway might transport proteases that degrade the extracellular matrix at adhesion sites and thus facilitate cell-matrix adhesion disassembly. In addition, focal adhesion-associated exocytosis may be more broadly important for extracellular matrix remodeling and play important roles in the dynamics of both normal and pathological tissue. They postulate that other cargo important for exocytosis is probably transported along these focal adhesion-associated microtubule tracks, and it will be important to determine to what extent this pathway is involved in three-dimensional cell migration systems that better represent actual physiology.
Research paper
Additional resources
Stehbens S, Pemble H, Murrow L, Wittmann T. 2012. Imaging intracellular protein dynamics by spinning disk confocal microscopy. Methods Enzymol 504:293-313.
Stehbens SJ, Wittmann T. 2014. Analysis of focal adhesion turnover: A quantitative live-cell imaging example. Methods Cell Biol 123:335-346.
Ettinger A, Wittmann T. 2014. Fluorescence live cell imaging. Methods Cell Biol 123:77-94.
Oreopoulos J, Berman R, Browne M. 2014. Spinning-disk confocal microscopy: present technology and future trends. Methods Cell Biol. 123:153-175.
