Resources
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Expand
Collapse
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Confocal microscopy is now a routinely used optical technique across a wide range of biological sciences, from plant science to mammalian models. Its use is popular because it has several benefits over conventional widefield microscopy:
In conventional widefield microscopy, the specimen is bathed in the light used to excite the fluorophores.
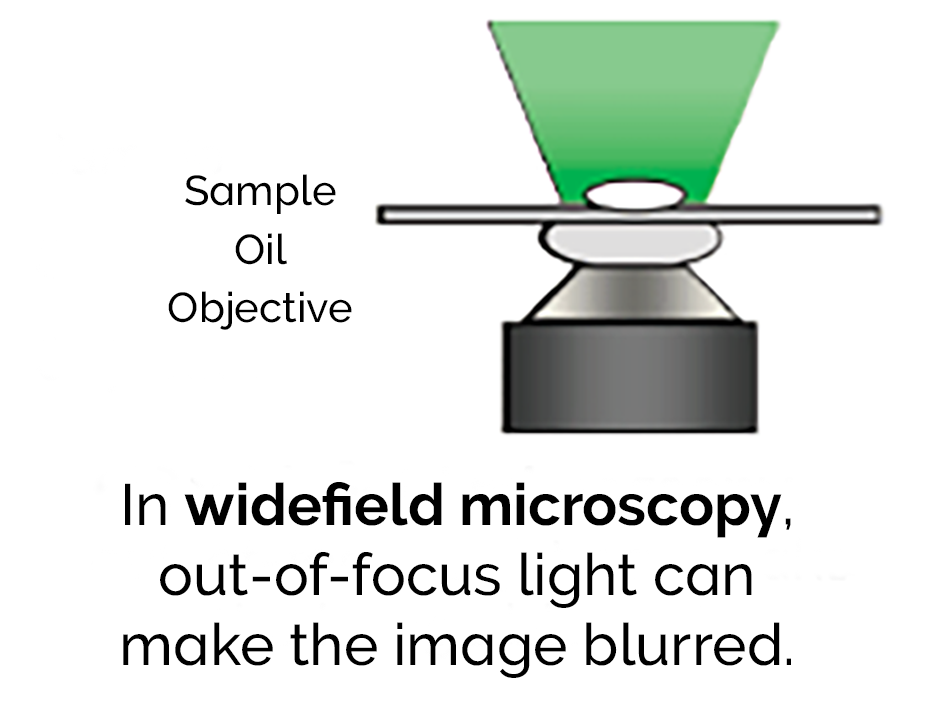
The fluorescence emitted by the specimen outside the focal plane of the objective interferes with the resolution of in-focus features. As the sample increases in thickness, the out-of-focus signal becomes increasingly dominant over the fine detail resulting in a blurred image.
In confocal microscopy, a pinhole is used to reject out-of-focus light. Only light from one plane is in focus and able to pass through the pinhole, as the name suggests 'confocal'.
This substantially improves both lateral and axial resolution. Which means that confocal microscopy is one of the best current imaging techniques.
There are several methods to exploit the pinhole effect. One is by using a laser which has a tightly focussed beam. This is scanned across the sample through a pinhole as shown in Figure 1b. To enhance the effect further and obtain the highest resolution, a disk pierced with thousands of pinholes spins and the simultaneous beams illuminate the sample to produce a sharp clear image.
The most common confocal technology is confocal laser scanning microscopy (CLSM). The sample is illuminated by a single point of light from a laser. The laser beam is scanned point by point in a raster pattern (regular parallel beams as on a TV screen). The signal is detected sequentially from each point by a photomultiplier tube until an entire image is created. 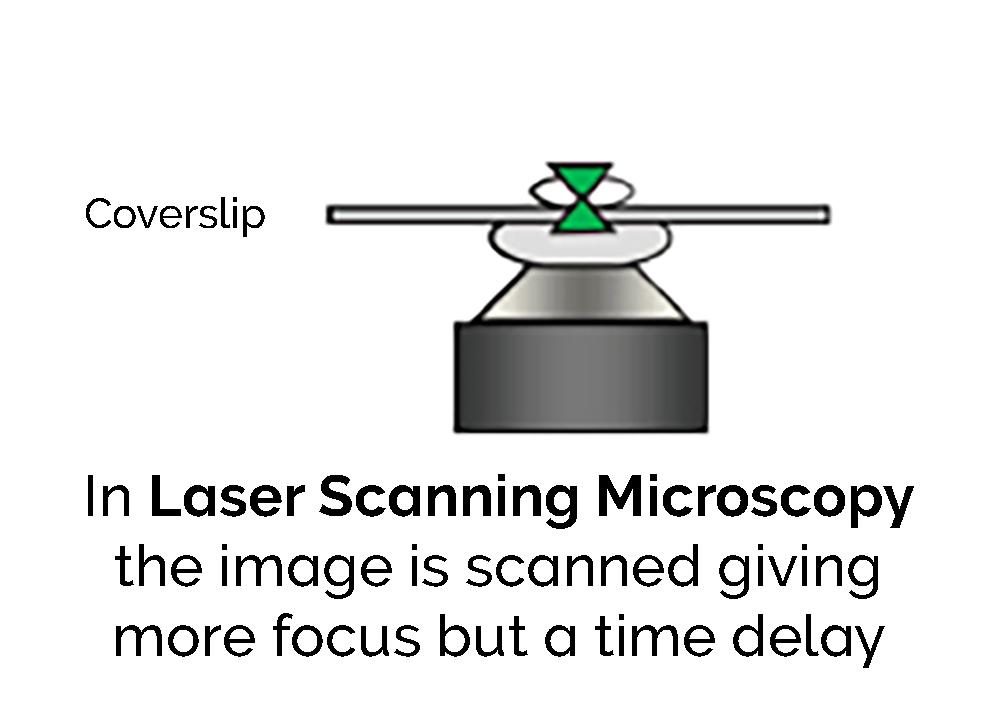
With CLSM, there is a trade-off between image resolution and speed. If the array consists of a 512x512 pixel array and each point is illuminated for 1 microsecond, then each scan will take about 262 milliseconds. The signal from each point must be acquired in that 1 microsecond and there is time delay of 0.26 second between the first and last points in the scan. Because the illumination time is brief, an intense laser beam is required. Furthermore, if the specimen is dynamic the time delay or skew can lead to errors in observation.
Spinning disk confocal laser microscopy (SDCLM) overcomes the problems with CLSM by exploiting the multiplex principle. The sample is illuminated and detected at multiple points simultaneously.
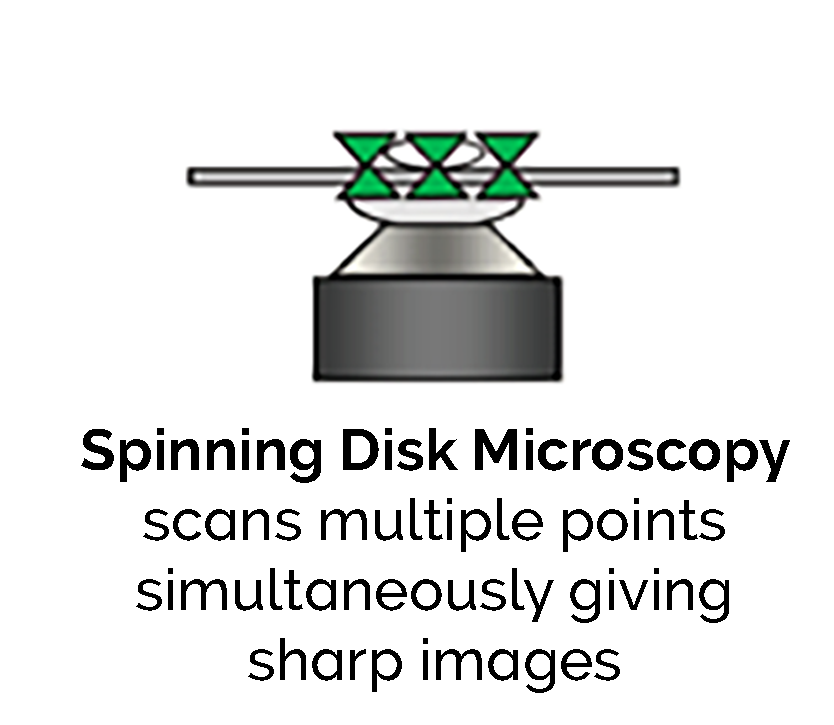
This work was originally carried out by Felgett in spectroscopy. It shows that using parallel detection delivers enhanced sensitivity. There is a publication by Wang [1] which provides a comparison of point and disk scanning systems for imaging live-cell specimens.
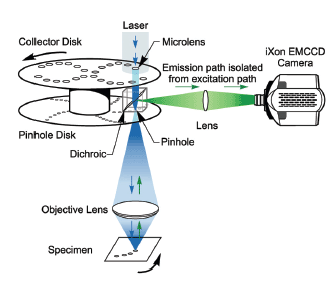
This figure shows how the dual spinning disk confocal laser scanner operates. This is the advanced version of the technology used in the Andor Dragonfly. In SDCLM an expanded beam illuminates an array of microlenses arranged on a top collector disk. Each microlens has an associated pinhole on a second pinhole disk below, positioned at the focal plane of the microlenses. The disks are joined by a shaft and rotated at high speed by an electric motor.
The scanner is part of the microscope system with the pinhole disk located in its primary image plane. An array of focused laser beams scans across the specimen. The technology was invented by the company Yokogawa [2]. Andor's Dragonfly Confocal takes this to the next level by improving the throughput, the homogeneity of illumination and pinhole spacing. It handles a wide variety of specimens from single molecules, to yeast to 3D model organs or organoids.
The pinholes and microlenses are arranged in a pattern, which scans a field of view that depends on the aperture size and the microscope objective magnification. The scanning laser beams excite fluorescent labels in the specimen. Fluorescence emission will be most intense where this array is focused - the focal plane. Some fraction of this light will return along the excitation path where it will be preferentially selected by the same 'confocal' pinholes. A dichroic mirror, which reflects emission wavelengths, is located between the two disks. This separates the laser emission from any excitation light reflected or scattered from the microscope optics. The geometry of the emission path results in a confocal fluorescence signal with extremely low background noise.
A further limitation in conventional CSLM is the use of photomultiplier tubes (PMTs) which are inefficient detectors as their quantum efficiency (QE, the probability of converting a photon to an electron) is rather low - typically 30-40%. In contrast, the spinning disk technique uses a camera as a detector. This can have a very high QE; e.g. Andor's iXon+ 897 EMCCD has a peak QE of more than 90%, making it a near-perfect detector. This combination of the dual spinning disk and high QE detector delivers a confocal instrument that can run at high speed and with unequalled signal-to-noise ratio (SNR). This is what we have with the Andor Dragonfly – beautiful sharp images, taking your microscopy to the next level.
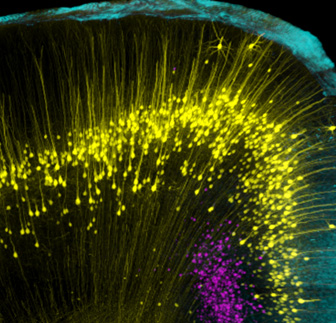
Mouse brain image taken with an Andor Dragonfly confocal microscope showing individual neurons. By Dr Hong Wei Dong, UCLA Department of Neurobiology. Explore further in our Whitepaper: Why Your Next Confocal Should Be Dragonfly
Wang E, et al. Performance comparison between the high-speed Yokogawa spinning disc confocal system and single-point scanning confocal systems. J. Microscopy. 2005;218(2):148–159.
www.yokogawa.com/uk/solutions/products-and-services/life-science/spinning-disk-confocal/#Details
